BLOG
ISO 9001:2015 Documentation Requirements
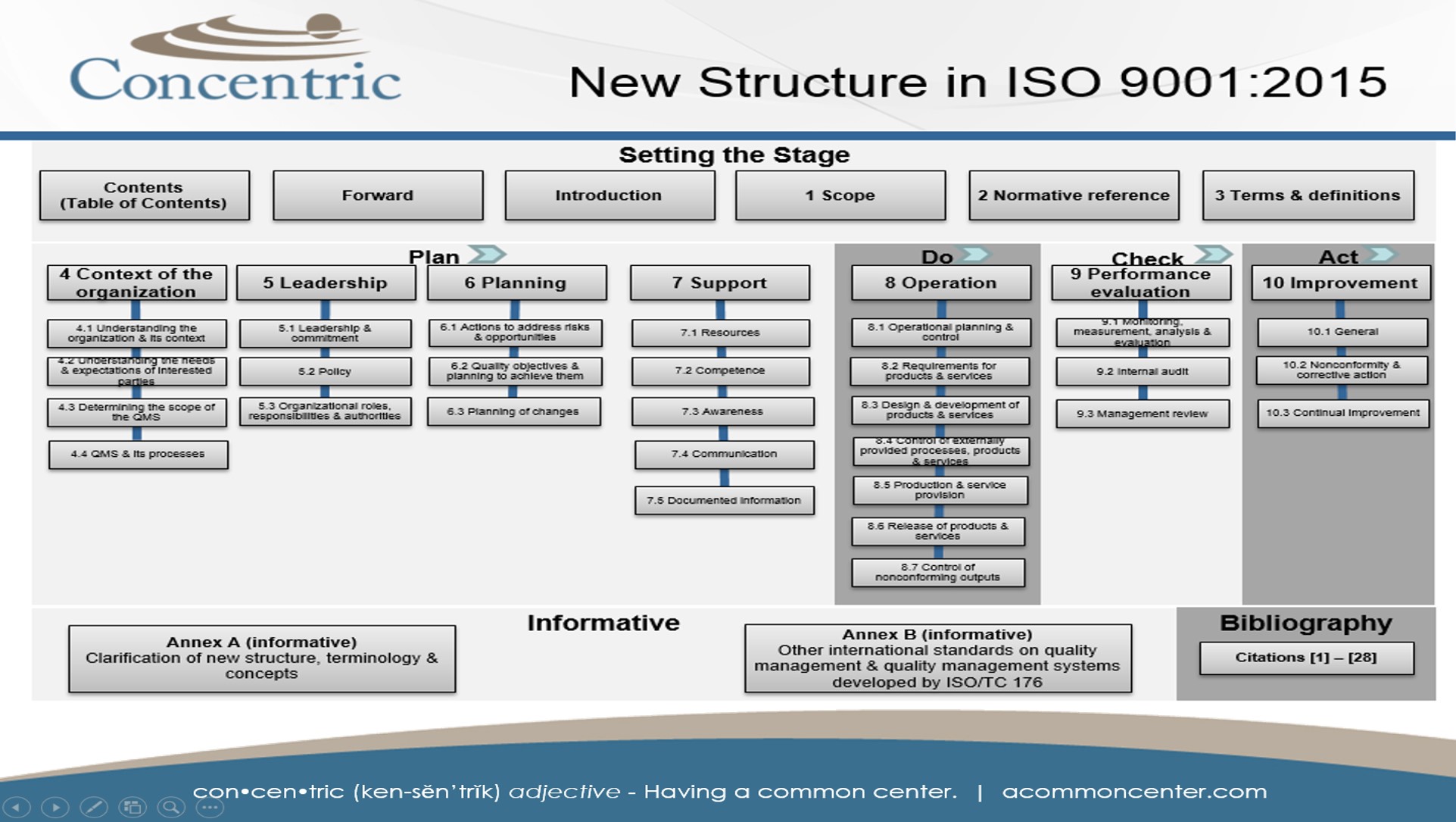
Here at Concentric, we like change and typically embrace it. However, we are in agreement that several changes to the 2015 release of the ISO 9001 standard are just annoying. Changes that make understanding of the requirements less simple are, well… not simple. One example is the ambiguity associated with how many and what kinds of documented procedures and records are required. Now that the term "documented information" is being used, subscribers have to get out their ibuprofen, highlighters and dig down into the standard looking for clues like the words maintained (document required) and retained (record required). We've decided to create some more user-friendly tools to help you to better understand what's inside this 42-page document.
The following sections may be helpful in better understanding what is required vs. what is optional, for those of us who appreciate simple. All documented information, be it a traditional document or record, must be controlled in accordance with clause 7.5 Documented information. In order to make implementation and auditing simple, we recommend purchasing a printed copy of the standard and highlighting where the terms “maintained” and “retained” exist. The following sections can also be used as a quick reference guide to assist you in locating documentation requirements, examples of documents that may be used “as needed” and matrices to help you map standard references to your own language.
Is your organization ready to adopt ISO 9001:2015?
If your answer is YES, which best describes the motivation for this adoption:
- This Standard is your industry’s general practice?
- It is your customer’s request?
- It is a Marketing initiative to improve your competitiveness?
- Or simply because you are ISO 9001:2008 and want to keep the registration?
You may acknowledge one or more of the justifications above as genuine enough in your case. However, your organization’s leadership truly believes that the very first statement of the ISO 9001:2015, clause 0.1 Introduction, is valid, and that’s significantly larger than all the justifications above combined!
“The adoption of a quality management system is a strategic decision for an organization that can help to improve its overall performance and provide a sound basis for sustainable development initiatives”.
The understanding of this Standard’s requirements, recommendations and permissions demonstrates an intuitive and logical rational that concludes that none of the requirements are unhelpful or useless. Indeed, compliance with ISO 9001:2015, means the minimum applied efforts and resources to achieve a better business structure, with more productivity, more competitiveness and, sure, more growth. Let us explain this rational!